What are the newest guidelines for measles vaccinations?
- Adults with no evidence of immunity should get 1 dose of MMR. Immunity is defined as documented receipt of 1 dose, or 2 doses, 4 weeks apart if high risk, of live measles virus-containing vaccine, laboratory evidence of immunity or laboratory confirmation of disease, or birthdate before 1957.
- High-risk people, including healthcare personnel, international travelers and students at post-high school educational institutions, should receive 2 doses.
- Persons who previously received a dose of MMR vaccine in 1963–1967 and are unsure which type of vaccine it was, or if it was an inactivated measles vaccine, should be revaccinated with either 1 (if low-risk) or 2 (if high-risk) doses of MMR vaccine. At the discretion of the state public health department, anyone exposed to measles in an outbreak setting can receive an additional dose of MMR vaccine even if they are considered complete for their age or risk status.
Why does a birthdate prior to 1957 confer immunity to measles?
People born before 1957 lived through several years of epidemic measles before the first measles vaccine was licensed in 1963. As a result, these people are very likely to have had measles disease. Surveys suggest that 95% to 98% of those born before 1957 are immune to measles. Persons born before 1957 can be presumed to be immune. However, if serologic testing indicates that the person is not immune, at least 1 dose of MMR should be administered.
Why is a second dose of MMR necessary?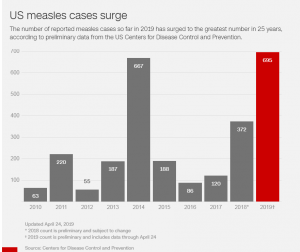
Between 2% and 5% of people do not develop measles immunity after the first dose of vaccine for a variety of reasons. The second dose is to provide another chance to develop measles immunity for people who did not respond to the first dose.
Are there any situations in which more than 2 doses of MMR are recommended?
There are two circumstances when a third dose of MMR is recommended, according to ACIP.
- Women of childbearing age who have received 2 doses of rubella-containing vaccine and have rubella serum IgG levels that are not clearly positive should receive 1 additional dose of MMR vaccine (maximum of 3 doses). Further testing for serologic evidence of rubella immunity is not recommended. NOTE: MMR should not be administered to a pregnant woman.
- Persons previously vaccinated with 2 doses of a mumps virus–containing vaccine who are identified by public health authorities as being part of a group or population at increased risk for acquiring mumps because of an outbreak should receive a third dose of a mumps virus–containing vaccine (MMR or MMRV) to improve protection. More information is available at www.cdc.gov/mmwr/volumes/67/wr/pdfs/mm6701a7-H.pdf
Many people age 60 years and older do not have records indicating what type of measles vaccine they received as children in the early 1960s. What measles vaccine was most frequently given in that time period? That guidance would assist many older people who would prefer not to be revaccinated.
Both killed and live attenuated measles vaccines became available in 1963. Live attenuated vaccine was used more often than killed vaccine. Without a written record, it is not possible to know what type of vaccine an individual may have received.
- The killed vaccine was found to be not effective and people who received it should be revaccinated with live vaccine.
- Persons born during or after 1957 who received killed measles vaccine or measles vaccine of unknown type, or who cannot document having been vaccinated or having laboratory-confirmed measles disease, should receive at least 1 dose of MMR.
- Some people at increased risk of exposure to measles (such as healthcare professionals and international travelers) should receive 2 doses of MMR separated by at least 4 weeks.
Do people who received MMR in the 1960s need to have their dose repeated?
Not necessarily.
- People who have documentation of receiving live measles vaccine in the 1960s do not need to be revaccinated.
- People who were vaccinated prior to 1968 with either inactivated (killed) measles vaccine or measles vaccine of unknown type should be revaccinated with at least one dose of live attenuated measles vaccine. This recommendation is intended to protect people who may have received killed measles vaccine which was available in the United States in 1963 through 1967 and was not effective (see above).
- Persons vaccinated before 1979 with either killed mumps vaccine or mumps vaccine of unknown type who are at high risk for mumps infection (such as persons who work in a healthcare facility) should be considered for revaccination with 2 doses of MMR vaccine.
 Please explain the Advisory Committee on Immunization Practices (ACIP)’s revised definition of evidence of immunity to measles, rubella, and mumps.
Please explain the Advisory Committee on Immunization Practices (ACIP)’s revised definition of evidence of immunity to measles, rubella, and mumps.
In the 2013 revision of its MMR vaccine recommendations, ACIP includes laboratory confirmation of disease as evidence of immunity for measles, mumps, and rubella. ACIP removed physician diagnosis of disease as evidence of immunity for measles and mumps. Physician diagnosis was previously not accepted as evidence of immunity for rubella. The decrease in measles and mumps cases over the last 30 years has made the validity of physician-diagnosed disease questionable. In addition, documenting history from physician records is not a practical option for most adults. The 2013 MMR ACIP recommendations are available at www.cdc.gov/mmwr/pdf/rr/rr6204.pdf
What can be done for unvaccinated people who have already been exposed to measles, mumps, or rubella?
The measles vaccine, given as MMR, may be effective if given within the first 3 days (72 hours) after exposure to measles. Immune globulin may be effective for as long as 6 days after exposure. Post-exposure prophylaxis with MMR vaccine does not prevent or alter the clinical severity of mumps or rubella. However, if the exposed person does not have evidence of mumps or rubella immunity they should be vaccinated since not all exposures result in infection.